Endermologie - First Cellulite Treatment to be FDA Approved
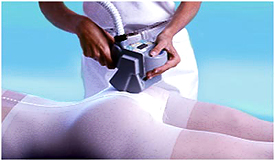 Treatments for cellulite remain largely unsuccessful because they only target the appearance of cellulite. It is finally understood that the dimpling on the surface of the skin was but a symptom of the underlying cause and to banish the bumps would require a deeply acting treatment.
Treatments for cellulite remain largely unsuccessful because they only target the appearance of cellulite. It is finally understood that the dimpling on the surface of the skin was but a symptom of the underlying cause and to banish the bumps would require a deeply acting treatment.
Leading the way is this field; Endermologie® is non-surgical and non-invasive. It involves the use of a motorised device with two adjustable rollers and controlled suction, which creates a symmetrical skin-fold. The skin gently folds and unfolds under the continuous action of the rollers allowing for smooth and regulated deep tissue mobilisation. As the viscosity of the subcutaneous fat layer decreases, blood flow and lymphatic drainage increase, facilitating the elimination of excess fluid and metabolites, while improving overall cellular function.
Endermologie, a sub dermal approach to treating cellulite, was pioneered by LPG Systems in France over a decade ago. Since its introduction to the USA, Endermologie was the first to be FDA approved for temporarily reducing the appearance of cellulite. Its safety and efficacy has been the subject of several clinical trials and on-going research reveals positive results.
Cellulite is a common term used to describe superficial pockets of trapped fat, which cause uneven dimpling or “orange peel” skin. It appears in 90% of post-adolescent women and is rarely seen in men. Common but not exclusive areas where cellulite is found are the thighs, buttocks, and the abdomen. Contrary to popular belief, cellulite is not related to obesity, since it occurs in overweight, normal, and thin women.
ADVERTISEMENT
——-

